Wednesday, 16 May 2007 - 10:40 AM
Auditorium (100) (Pfahler Hall)
28
Terminal Olefins through Cobalt-Catalyzed Alkene Dimerization
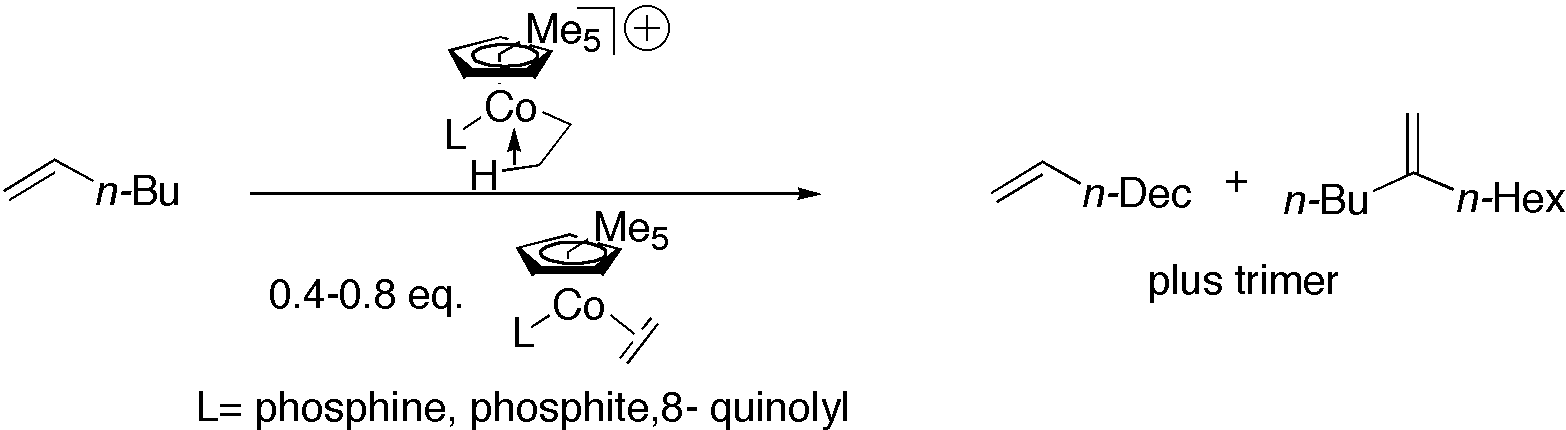
[Cp*P(OMe)3CoCH2CH3]+ [BArF]-, generated by the addition of HBArF to Cp*P(OMe)3Co(ethene) catalyzes the oligomerization of 1-hexene to give dimers and trimers. When a deficit of the acid is used, linear &alpha olefin dimers are produced at the expense of trimeric products: e. g. 1-butene, 1-hexene, and 1-octene give 1-octene, 1-dodecene, and 1-hexadecene, respectively. The size of the phosphine ligand determines the ratio of linear to branched dimer formed in the reaction. We will discuss a novel mechanism for the reaction and the synthesis of several new complexes designed to favor linear over branched products in this reaction.
Back to Cope Scholars Award Symposium I: Michael P. Doyle
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)