Wednesday, 16 May 2007 - 2:55 PM
106 (Pfahler Hall)
118
Beta-Propiolactone Inactivation of HIV for Vaccines
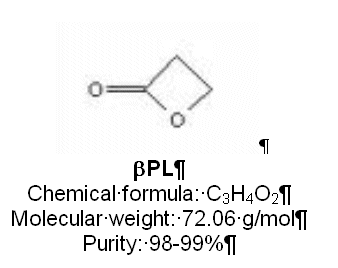
Betapropiolactone (βPL) has been used historically as a highly effective disinfectant against a variety of bacteria, fungi, and viruses. It is currently utilized to inactivate multiple viral agents to produce licensed human vaccines, including Influenza and Rabies. We will discuss an efficient, reproducible, and scalable use of this small ring heterocyclic compound used for the first inactivation step of an investigational HIV-1 therapeutic vaccine currently in clinical trials. The large scale qualified �"PL process outlined here may be of use to others in meeting viral safety requirements for the production of future vaccines. Basic chemistry, safe handling, GMP bioprocess/quality considerations, scale up and performance qualifications, and technical challenges will be discussed.
Back to Bioprocess Science/Biomanufacturing Session
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)