Wednesday, 16 May 2007 - 1:50 PM
Auditorium (100) (Pfahler Hall)
131
Synthesis of Macrocycles via Cobalt-Mediated [2 + 2 + 2] Cycloadditions
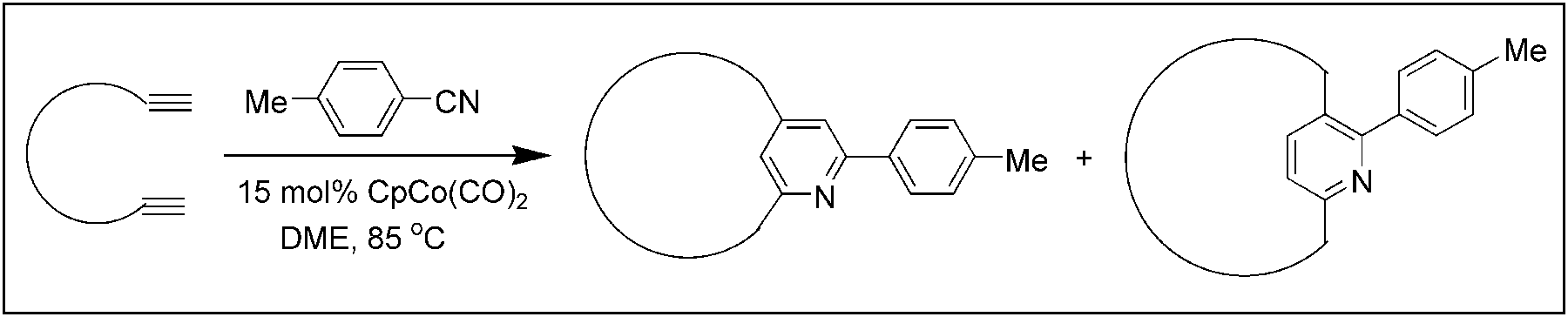
We have investigated the formation of macrocycles a,w-diynes in cobalt(I)-mediated co-cyclotrimerization rections. In these [2 + 2 + 2] cycloadditions, a,w-diynes were reacted with nitriles, cyanamides, or isocyanates in the presence of CpCo(CO)2 to yield pyridine-containing macrocycles, in the form of meta- and para-pyridinophanes. E.g., a long-chain a,w-bis-alkyne reacts with p-tolylnitrile to give a 1:1 mixture of meta- and para-pyridinophanes in 50-60% yield (see graphic). We developed an improved reaction protocol that offers a convenient, flexible synthetic approach, without photo-irradiation or syringe-pump addition. This [2 + 2 + 2] cycloaddition process has excellent atom-economy and supplies substantial molecular complexity in a single step. Thus, we synthesized some macrocyclic bis(indolyl)maleimides ("multiheterophanes") as potential protein kinase inhibitors. Some of these derivatives proved to be potent, selective inhibitors of glycogen synthase kinase-3b (GSK-3b). An X-ray structure of an inhibitor ligand complexed with GSK-3b will be presented.
Back to Cope Scholar Award Symposium II: Franklin A. Davis
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)