Wednesday, 16 May 2007 - 2:30 PM
Auditorium (100) (Pfahler Hall)
132
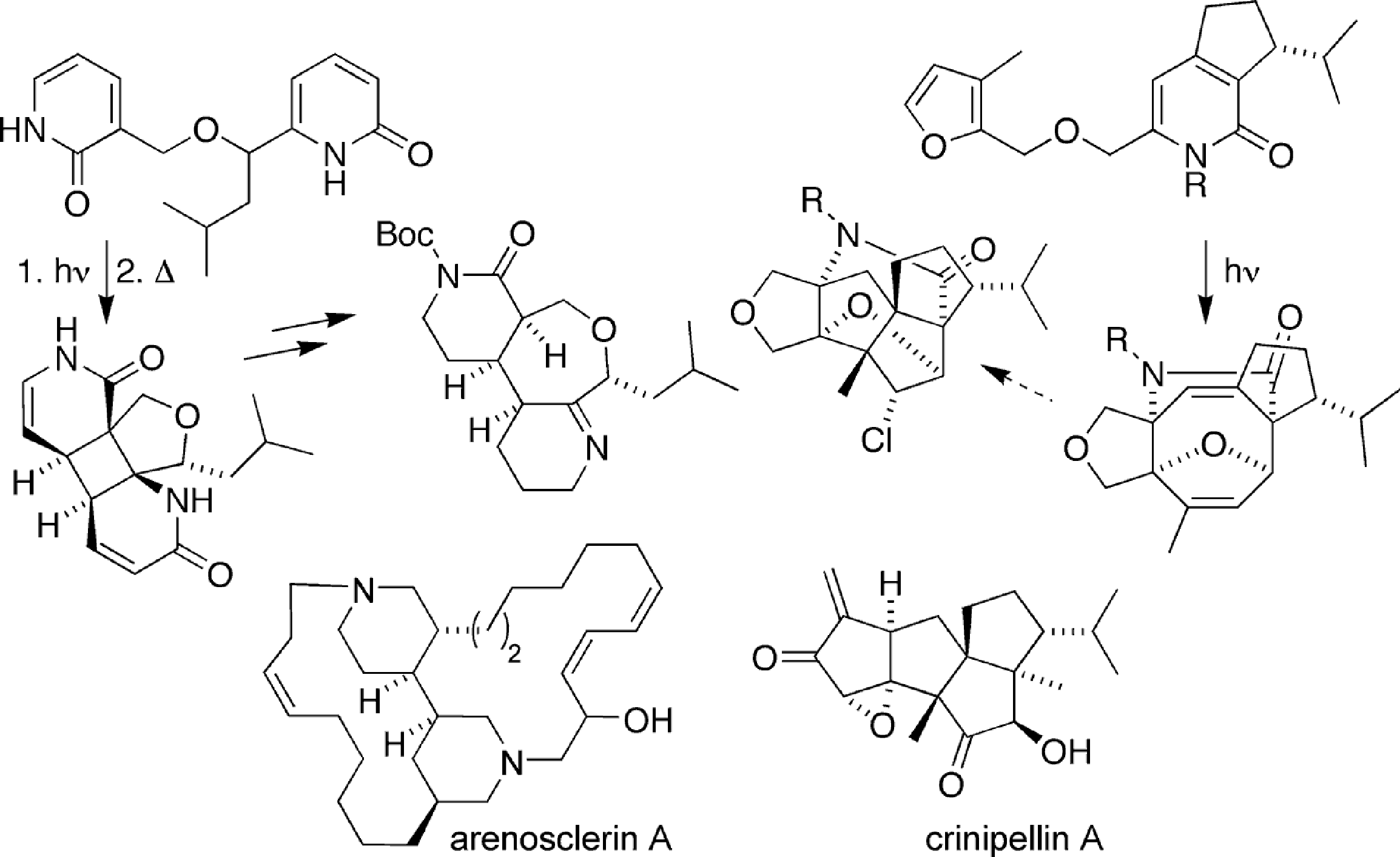
Complexity via [4+4] Photocycloadditions
Application of the efficient [4+4] photocycloaddition of 2-pyridones to natural product synthesis, studied for cyclooctane ring structures, has been adapted to additional polycyclic systems. A [4+4] cycloaddition – [3,3] rearrangement – and retro-Mannich sequence assembles unsymmetrically coupled piperidines with predictable stereochemistry. In a second model study, intramolecular [4+4] cycloaddition of a pyridone and a furan yields a pentacyclic cyclooctadiene. The transannular cyclization of this intermediate can be closed to the tetraquinane framework of the crinipellin.
Back to Cope Scholar Award Symposium II: Franklin A. Davis
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)