Wednesday, 16 May 2007 - 11:30 AM
012 (Pfahler Hall)
15
Atropisomeric Cobaloximes: synthesis and NMR study
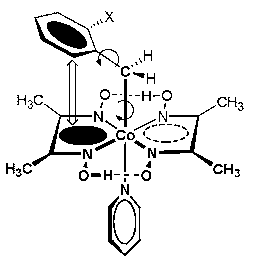
The spectral and structural properties of cobaloximes and NMR, in particular, have been extensively used. In majority of the complexes, the dmgH (Me) signal appears as a sharp singlet at »2.0 ppm in the 1H NMR spectra indicating the chemical equivalence of all four methyl groups. Non-equivalence of the dmgH in 1:1 ratio, however, has been observed in a few cases, when either of the axial ligands is asymmetric.
The variable temperature NMR study in 2-substituted benzyl cobaloximes will be discussed. The cobalt bound CH2 becomes diastereotopic and the dmgH methyl protons show non-equivalence (1:1) at sub-zero temperature. The non-equivalence is quite striking since there is no chiral group attached to the cobalt. The interaction of the axial with the equatorial ligand causes hindered rotation of the Co–C and /or C–Ph bond and leads to nonequivalence. This is partly due to the weak interactions between the benzyl group and the dioxime ring current and is supported by the crystal structures of benzyl cobaloximes.
Related References: B. D. Gupta et al
Organometallics, 26 (2007) 658; Organometallics, 25 (2006) 92; Organometallics, 24 (2005) 4454; Organometallics, 24 (2005) 1501; Organometallics, 23 (2004) 2069
Web Page: home.iitk.ac.in/~bdg/
Back to Inorganic Chemistry General Session
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)