Wednesday, 16 May 2007
3rd Floor Hall (Pfahler Hall)
54
Reaction of dimethyl sulfoxide with isatoic anhydrides. Isolation of unexpected rearrangement products
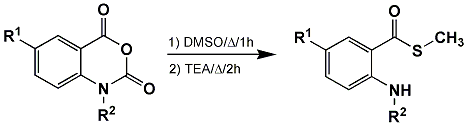
Abstract: During the investigation of a novel o-aminoaryl oxazoline synthesis from the reaction of N-substituted isatoic anhydrides and 2-chloroethylamine hydrochloride in DMSO using an equimolar quantity of base, o-amino thiomethyl esters were observed as side products. In some cases, an o-amino thiomethyl ester was the major product. A general mechanism for the formation of these thioesters is proposed. Studies to support the proposed mechanism will also be discussed.
Back to Poster Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)