Friday, 18 May 2007 - 10:10 AM
210 (Pfahler Hall)
411
Synthesis of a Conformationally Constrained Collagen-like Polypeptide Mimic
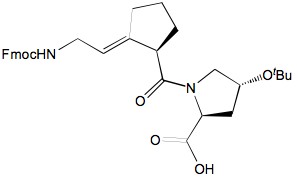
Collagen is one of the most abundant and important proteins in nature. The primary structure of collagen can be represented as (Xaa-Yaa-Gly)n, where 10% of Xaa is praline(Pro), and 10-12% of Yaa is hydroxyproline(Hyp). A side-chain protected monomer mimic, Fmoc-Gly-[?(E)CH=C]-Pro-Hyp(tBu)-OH with a conformationally constrained prolyl amide bond was designed and synthesized. Since proline residues in collagen are in the trans-conformation, the (E)-alkene monomer was synthesized stereoselectively. And the desired stereo-isomer was also separated to mimic the natural all L-amino acid residues. The synthesized tripeptide mimic is ready for solid phase synthesis.
Back to Organic Chemistry General Session II
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)