Wednesday, 16 May 2007
3rd Floor Hall (Pfahler Hall)
57
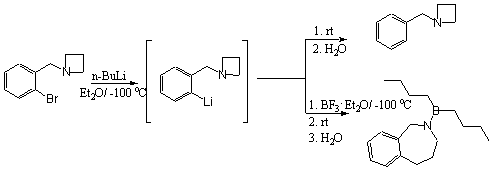
Lewis acid - mediated ring expansion of azetidines to benzazepines
The major goal of this project is to synthesize the biologically active compound 2,3,4,5-Tetrahydro-1H-2-benzazepine from ortho-bromobenzylazetidine. This research focuses on the use of halogen-metal exchange chemistry in order to create a nucleophilic carbanion at the bromine-bound-carbon on the benzene ring. This carbanion in theory should open the azetidine ring, ultimately resulting in the formation of 2-benzazepine. Initial NMR and mass spectral data, as well as elemental analysis, indicate that with the use of a strong Lewis acid, the azetidine can be activated to undergo ring expansion to form the final benzazepine compound indicated below.
Back to Poster Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)