Wednesday, 16 May 2007 - 10:40 AM
106 (Pfahler Hall)
39
Contributions of the 8-methyl group normal modes to the flavin vibrational spectrum
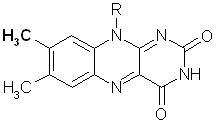
The flavin molecule is ubiquitous throughout biochemistry as a cofactor of various enzymes,[1] and the interaction of its 8-methyl group with the protein may be an important factor in regulating its function.[2] In this study, the hydrogens of the 8-methyl group of the flavin were exchanged for deuteriums, and the changes to the NMR and Raman spectra were noted throughout the procedure. Further information was provided by a DFT calculation of the theoretical vibrational spectra and vibrational modes. Several unexpected changes occurred as a result of the deuteration, which are all clearly explained as resulting from the interplay of mass and decoupling effects, the evidence for which comes from the combined information from the theoretical calculations, experimental Raman spectra, and NMR spectra. This study not only provides information on the identification of the 8-methyl group interactions, but also a general method by which methyl group vibrations can be better understood.
1 S. Weber, Biochimica et Biophysica Acta 1707, 2005, pp. 1
2 A. Mees, T. Klar, P. Gnau, U. Hennecke, A.P.M. Eker, T. Carell, L-O Essen, Science, 306, 2004, pp. 1789-1793
Back to Physical Chemistry General Session
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)