Friday, 18 May 2007 - 11:10 AM
210 (Pfahler Hall)
414
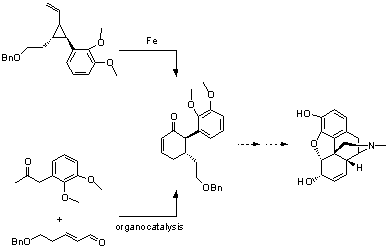
Toward the total synthesis of morphine: The asymmetric construction of 5-alkyl, 6-aryl cyclohexenones
We are developing methods for the asymmetric construction of 5-alkyl,6-aryl cyclohexenones for use in natural product synthesis. We are exploring two approaches: The Fe mediated ring expansion of a vinylcyclopropane, and an organocatalyic Robinson annulation of an aryl ketone with an a,b�unsaturated aldehyde. Our objective is the total synthesis of morphine.
Back to Organic Chemistry General Session II
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)