Wednesday, 16 May 2007
3rd Floor Hall (Pfahler Hall)
68
A novel route to tetra-substituted pyrazoles using CAN
Functionalized pyrazoles are playing an increasingly important role in the development of new pharmaceuticals and have established their place in the agrochemical industry. The drug celecoxib, antidiabetic agents, antitumor agents, kinase inhibitors, and HIV-1 reverse transcriptase inhibitors all contain pyrazole motifs. Recently, 4-alkyl-1,3,5-triarylpyrazoles have been examined in detail because of their potential biological activity. More specifically, propylpyrazole triol (PPT) has been shown to be an equivalent to β-estradiol as a ligand for the estrogen receptor (ER). In the present work, a method using ceric ammonium nitrate (CAN) for synthesizing PPT as well as other ER ligand candidates has been developed through allylation of β-diketones and subsequent reaction with various substituted hydrazines. Experimental results show that a wide range of aliphatic and aromatic substituted diketones can be used with this method. In unsymmetrical β-diketones, steric hindrance provides the driving force for selectivity. The total synthesis of PPT using this method provides an overall isolated yield of 30.4%. 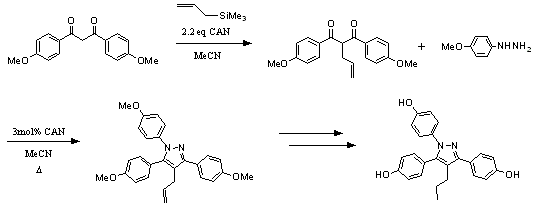
Back to Poster Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)