Wednesday, 16 May 2007
3rd Floor Hall (Pfahler Hall)
71
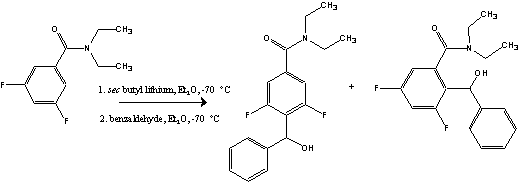
A regioselective study of the metalation of N,N-diethyl-3,5-difluorobenzamide
Substituted benzamides are widely used as intermediates in synthetic organic chemistry, and understanding the influence of substituents on the directing power of the benzamide in metalation reactions is critical. Previously, studies in this laboratory group have shown the metalation of tertiary 3,5-dichlorobenzamides resulted in only para-substituted products rather than the expected ortho-substituted products. To further continue this research, N,N-diethyl-3,5-difluorobenzamide was synthesized and metalated with benzaldehyde as an electrophile. Results have shown that para-substituted products are primarily favored, but it is suspected that ortho-substituted addition products have also been formed.
Back to Poster Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)