Friday, 18 May 2007 - 11:30 AM
210 (Pfahler Hall)
415
Toward the total synthesis of ritterazine O
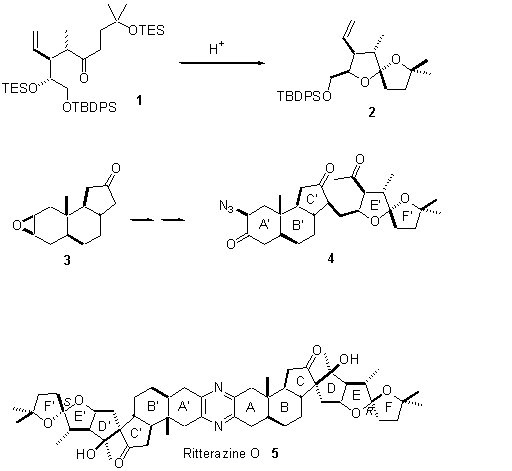
We have prepared the enantiomerically-pure 5/5-spiroketal 2 required for the synthesis of the ritterazines, by ring closure of 1 followed by equilibration with high diastereocontrol. Further, we have coupled the spiroketal with 3, and after a series of transformations, we have obtained 4. We will describe progress toward our target 5.
Back to Organic Chemistry General Session II
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)