Thursday, 17 May 2007 - 2:10 PM
210 (Pfahler Hall)
334
Synthesis and evaluation of PABA/NO analogs as anticancer leads
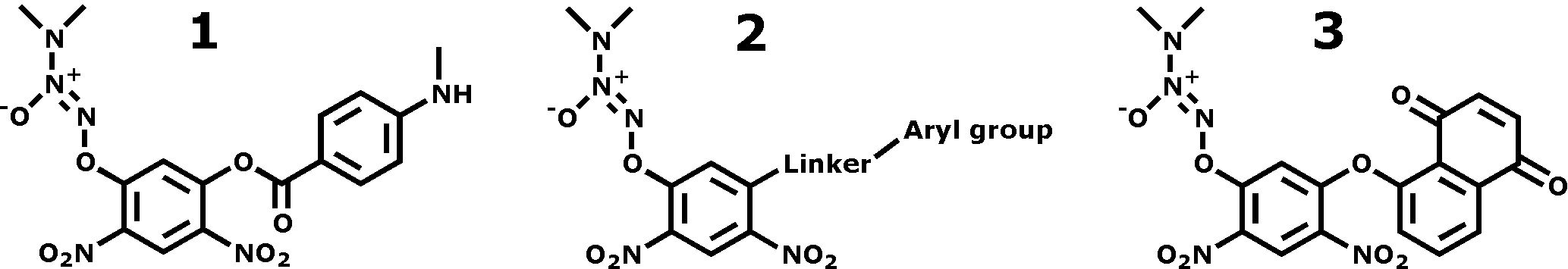
Many cancers overexpress the p-isozyme of glutathione transferase (GSTp), thus presenting an opportunity for selective targeting of cancer cells. Rational design of prodrugs intended to release cytotoxic levels of nitric oxide (NO) in GSTp-overexpressing cancer cells yielded PABA/NO (1), which inhibited the growth of ovarian cancer xenografts in mice with a potency similar to that of cisplatin. With the aim of reducing its susceptibility towards hydrolysis and free glutathione (GSH) attack, approximately 20 analogs were synthesized of the general structure 2. These analogs were assayed for both GSH-induced NO release and for their ability to inhibit HL-60 (leukemia) cell proliferation in vitro. An inverse correlation was observed between the quantity of NO released and IC50 value, supporting NO as the cytotoxic agent in these prodrugs. One exception was compound 3, which exhibits both low NO release and a low IC50 (2.6 μM). Possible mechanisms explaining 3's anticancer effects will be discussed.
Back to Organic Chemistry General Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)