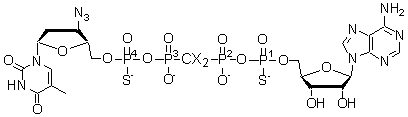Thursday, 17 May 2007 - 3:30 PM
012 (Pfahler Hall)
332
Application of Reverse Phase HPLC in Preparation and Configuration Identification of Hydrolysis Resistant AZTpSpCX2ppSA
AZT excision is the predominant mechanism of AZT drug resistance by HIV RT, and AZTppppA is the excision product. Its hydrolysis-resistant analogs, AZTpSpCX2ppSA (X = H or F) containing two chiral centers at both terminal phosphorus atoms, can be used to study the inhibition of excision and may be inhibitors of the excision reaction. An efficient method was developed to synthesize AZTpSpCX2ppSA (X = H or F) with total yields up to 80%. The four diastereomers of each product were separated and purified by reverse phase HPLC (three Waters Delta-Pak PrepPak� cartridges, C18, 300�, 40 mm x 100 mm, 15 mm). Enzymatic degradation of the diastereomers was conducted with snake venom phosphodiesterase and the degradation process was monitored by LC-MS (Waters Atlantis� dC18 column, 100�, 4.6 mm x 50 mm, 3.0 mm). The degraded components were identified by MS(ESI), UV and retention time. From formation rates of the degradation products, the configurations of diastereomers were identified, based on the fact that the [R]-diastereomer of a phosphothioate is degraded faster by phosphodiesterase than the [S]-diastereomer. From the digestion rates of the diastereomers, their stabilities to snake venom phosphodiesterase were compared, and the [SP1,SP4]-isomers were found to be the most stable. Furthermore AZTpSpCX2ppSA was found to be far more stable to snake venom than the natural AZTppppA. It was found that the eluting rates of the diastereomers from reverse phase HPLC are correlated with the digestion rates by snake venom, and the fastest eluting diastereomer was digested most slowly.
Back to Chromatography Forum of the Delaware Valley - Student Award Symposium
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)