Wednesday, 16 May 2007 - 2:30 PM
107 (Pfahler Hall)
108
Application of Resonance Energy Transfer to Study Binding of DNA Photolyase to UV-Damaged DNA
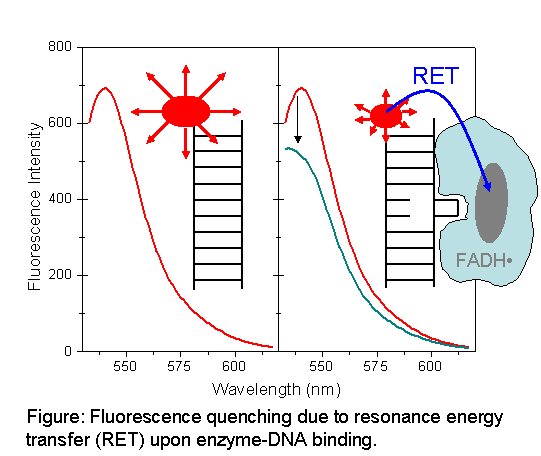
DNA photolyase is a highly efficient DNA repair enzyme that is specialized in the reversal of the UV-induced dimerization of adjacent pyrimidine bases. In the majority of the cases (80%), a cis,syn cyclobutane pyrimidine dimer (CPD) is formed upon the irradiation of DNA with UV-light. Arguably the most important step in DNA repair is the recognition of the lesion and binding of the DNA-repair enzyme(s) to the lesion for repair. In this paper, we present the application of resonance energy transfer to monitor the binding of DNA photolyase to CPD-containing DNA. We have used three purified DNA undecamers each with a single CPD lesion in a different location and complementary strands with either a fluorophore-quencher pair or only a fluorophore to monitor the binding process by using steady-state fluorescence and fluorescence stopped-flow spectroscopy. The three strands show similar equilibrium binding constants but different binding rate constants. These differences and the implications for the DNA-damage recognition step by CPD photolyase will be discussed.
Back to Biological Chemistry General Session
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)