Wednesday, 16 May 2007
3rd Floor Hall (Pfahler Hall)
91
Reactions of 2-Aminopyrroles with Electron-Deficient Heteroaromatic Azadienes
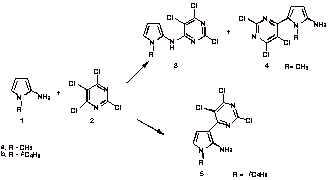
Reaction of 2,4,5,6-tetrachloropyrimidine (2) with 2-aminopyrroles (1) was studied as a possible route to azaindoles. Instead of cycloaddition substitution by addition-elimination occurred at both the exo amino group and C-5 or at C-3, depending on the size of the 1-alkyl substituent. Initial reaction of the 2-aminopyrrole with 2,4,5,6-tetrachloropyrimidine gave a zwitterion (Meisenheimer complex) intermediate. When the zwitterion contains a good leaving group substitution by addition-elimination competes successfully with cycloaddition.
Pyrrole 4 reacted with 2,4,6-tris(trifluoromethyl)-1,3,5-triazine to give a pyrrolo[2,3-d]pyrimidine cycloadduct. In contrast 5 was unreactive. This difference in reactivity was used to confirm the structures of 4 and 5. Only when a catalytic amount of base was present did pyrrole 4 react with the 1,3,5-triazine. A stable intermediate was detected by proton NMR. The expected cycloadduct was obtained upon addition of acid (TFA) to this intermediate. The effects of added base and acid, on the progress of the cycloaddition reaction, can best be explained by a cascade mechanism that has several reversible steps. This work was supported by a grant (CHE-0110801) from the National Science Foundation.
Back to Poster Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)