Thursday, 17 May 2007 - 2:50 PM
210 (Pfahler Hall)
336
Towards the Total Synthesis of Pregna-4,16-diene-3,11,20-trione
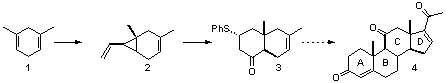
Symmetric diene 1, readily obtained via Birch reduction of m-xylene, allows easy access to the vinyl cyclopropane 2. Iron-mediated cyclocarbonylation followed by the addition of thiophenol affords the trans-fused steroidal C/D synthon 3. We will describe our continuing efforts toward the synthesis of pregna-4,16-diene-3,11,20-trione 4.
Back to Organic Chemistry General Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)