Thursday, 17 May 2007 - 3:50 PM
210 (Pfahler Hall)
338
Asymmetric Synthesis of Tetrahydroisoquinoline and Tetrahydroprotoberberine Derivatives from the Condensation of Laterally Lithiated Species with Sulfinimines
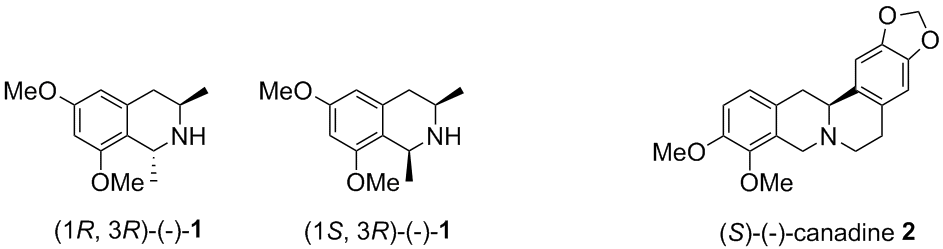
Recently there has been great interest from the scientific community to exploit the rich diversity of biological activity that 1,2,3,4-tetrahydroisoquinoline (THIQ) alkaloids possess. However, the discovery and development of a drug from this class of alkaloids has been circumscribed by the amount of material that can be extracted from natural sources and synthetic limitations. Conventional synthetic approaches for the construction of these alkaloids, such as the Bischler-Napieralski and Pictet-Spengler reactions, often suffer from unreliable regio- and stereoselectivity and undesirable side reactions involving the iminium ion intermediates. An alternative method for the asymmetric construction of THIQs is the addition of laterally lithiated species to enantiopure sulfinimines (N-sulfinyl imines) to afford sulfinamides. Facile removal of the N-sulfinyl auxiliary and cyclization affords the corresponding optically active THIQs with substitution patterns not easily accessible by other methods. This new method was applied to the asymmetric synthesis of cis- and trans-6,8-dimethoxy-1,3-dimethyl-1,2,3,4-tetrahydroisoquinoline 1, which is the THIQ segment of the potent anti-HIV alkaloids michellamines A and B, and (-)-canadine 2, which is one of the active ingredients in Golden Seal and is being investigated for a for a diverse range of biological activities. The scope and limitations of various directing metallation groups to facilitate ortho and lateral lithiation reactions and their concomitant condensation with sulfinimines during the aforementioned syntheses will be the topic of discussion.
Back to Organic Chemistry General Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)