Wednesday, 16 May 2007
3rd Floor Hall (Pfahler Hall)
95
Synthesis and Structure-Activity Relationship of Tricyclic Piperidinylidene- and Tropanylidene-Based Delta Opioid Agonists with Novel Delta Addresses
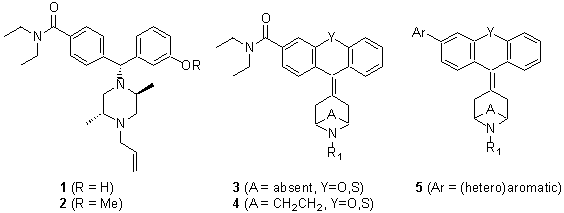
In recent years, targeting of the delta opioid receptor has emerged as a potential alternative for the treatment of a variety of painful and non-painful conditions. Following the discovery of the enkaphalin peptides as endogenous ligands for the delta opioid receptor, several selective peptidic ligands have been reported. Since then, non-peptide delta-selective opioid agonists such as BW-373U86 (1) and SNC-80 (2) have been studied extensively. Earlier, we reported on a series of tricyclic piperidinylidenes (3) and tropanylidenes (4) as novel classes of potent delta opioid receptor agonists. A prominent feature of the delta opioid pharmacophore is the N,N-diethylcarboxamide, commonly referred to as the �delta address'. As part of a structure-activity relationship study around 3 and 4, a number of interesting (hetero)aromatic replacements for the N,N-diethylcarboxamide delta address were identified. The synthesis and delta opioid binding affinity of this class of compounds will be presented.
Back to Poster Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)