Thursday, 17 May 2007 - 4:10 PM
210 (Pfahler Hall)
339
2-Alkyl cyclohexenones from aldehydes: synthesis of the Bachmann-Miescher Estrone intermediate
2-alkyl cyclohexenones are powerful intermediates for organic synthesis. The Wittig reaction of a series of aliphatic aldehydes with (cyclopropylmethyl)triphenylphosphonium bromide delivered the corresponding alkenyl cyclopropanes. UV radiation in the presence of Fe(CO)5 converted the alkenyl cyclopropanes to the 2-substituted cyclohexenones. Reaction with Mander's Reagent and methylation enabled a four-step synthesis of the tricyclic ketone that was the key intermediate in the Bachmann-Miescher estrone synthesis. 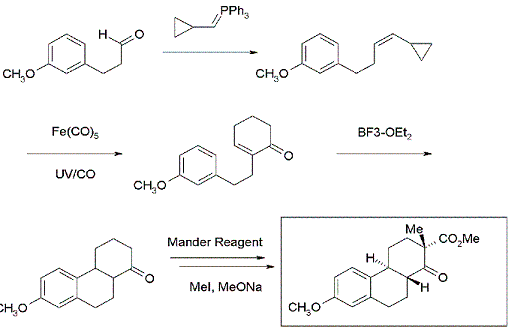

Back to Organic Chemistry General Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)