Wednesday, 16 May 2007
3rd Floor Hall (Pfahler Hall)
86
From high-throughput catalyst screening to reaction optimization: Detailed investigation of regioselective Suzuki cross-coupling of 1,6-naphthyridone dichloride
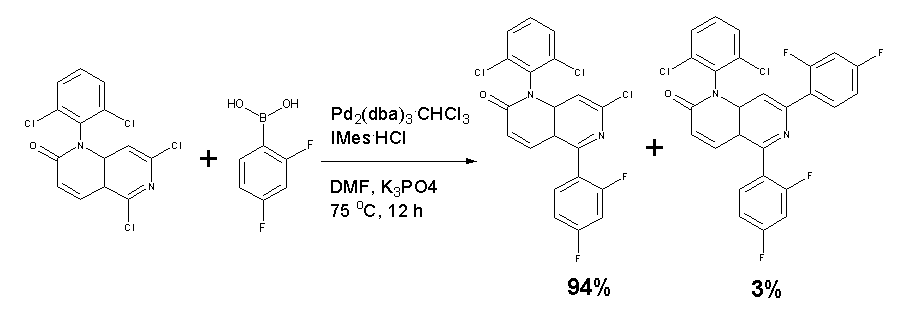
Efficient catalyst systems were discovered for the regioselective Suzuki cross-coupling of 1,6-naphthyridone dichloride through high-throughput catalyst screening and DOE optimization. With Pd2(dba)3.CHCl3 as the precatalyst, either (2-MeO-Ph)3P or IMes.HCl afforded >95% conversion to the coupling products with up to 92% desired regioselectivity. DMF/K3PO4 was found to be the most effective solvent and base. The concentration profiles of reactants and products indicated that with the regioselective catalyst, the first coupling step at one of the two competitive reactive centers was 10 times faster than the second coupling step at the other reactive center, resulting in high regioselectivity of the desired mono-adduct.
Back to Poster Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)