Thursday, 17 May 2007 - 4:30 PM
210 (Pfahler Hall)
340
Developing the structure-activity relationships in cADPR: Conformational analysis of cADPR analog agonists and antagonists
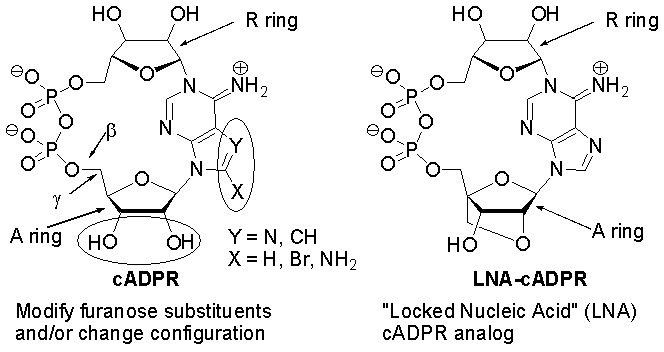
Cyclic adenosine diphosphate ribose (cADPR), a cyclic metabolite of NAD+, is a second messenger that causes release of calcium from intracellular stores. It is well established that the conformation of the furanose ring is profoundly affected by the configuration and identity of electronegative substituents at the 2'- and 3'-positions. Changes at the 2'- and 3'-positions in the adenosine furanose ring have produced both cADPR agonists and antagonists, whereas only antagonists result from changes at the 8-position of the adenine ring. Replacement of the adenine N7 with CH leads to an analog with greatly enhanced resistance to spontaneous hydrolysis. We will present our recent work on the NMR solution structures of these analogs, with a discussion of how the conformations of the agonists and antagonists differ.
Back to Organic Chemistry General Session I
Back to The Middle Atlantic Regional Meeting (May 16 - 18, 2007)