Tuesday, May 20, 2008 - 3:50 PM
Library Building, Rm LB-15 (Queensborough Community College)
479
Controlling Photoreactivity of Coumarins in Water Soluble Nano-Cavities
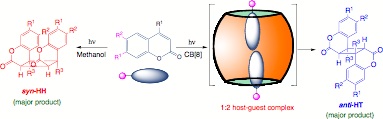
This paresentation will focus on ways of controlling photoreactivity of coumarin derivatives within cucurbit[8]uril (CB[8]) nano-cavities. Substituted coumarin derivatives that are either neutral (7-alkyl, 6-alkyl, 7-hyxoxy or 6-hydroxy) or cationic (7-ammonium or 6-amnonium) form 1:2 host-guest complex with CB[8], that are completely soluble in water and are characterized by NMR spectroscopy. Direct irradiation of these coumarin�CB [8] complexes in water gives anti-head-to-tail (anti-HT) adduct as the major product, which is again characterized by NMR spectroscopy. Preferential formation of anti-HT adduct within cucurbiturils, not generally observed upon direct irradiation in isotropic media, highlights the role of CBs in altering photoreactivity of coumarin derivatives. Selective formation of coumarin adducts (4-diffenent adducts are possible) in different organized assemblies is generally unpredictable. Based on reported crystal structure parameters and optimized structures for various coumarin adducts, it is speculated that the available free space within CB[8] nanocavity facilitates formation of the anti-HT 5. Employing CBs as a template is a powerful tool to control photoreactivity of various substituted coumarin derivatives leading to selective formation of anti-HT adduct, which is not generally observed in other isotropic media.
Web Page: sivagroup.chem.ndsu.nodak.edu/