Sunday, May 18, 2008 - 8:35 AM
Science Building, Rm S-112 (Queensborough Community College)
21
Synthesis of An Oligodeoxyribonucleotide Adduct of Mitomycin C by the Postoligomerization Method, Via a Triamino Mitosene
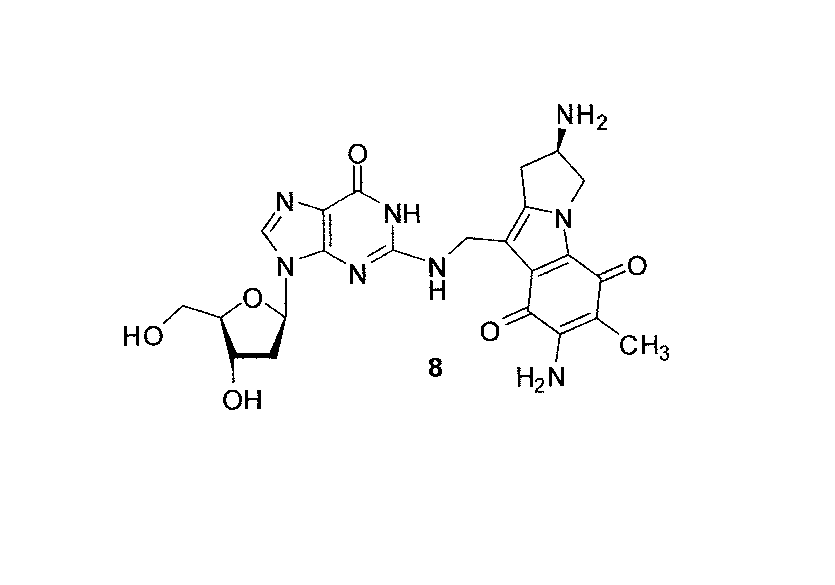
We report here the first alternative access to one of the DNA adducts of Mytomicin C, an antitumor antibiotic used in clinical cancer chemotherapy (MC), based on organic synthetic methods, featuring a postoligomerization. Specifically, we describe a synthesis of monoadduct 8 on both the nucleoside and oligonucleotide levels. MC has numerous sensitive functional groups and the adduct described herein is, to the best of our knowledge, the most complex DNA adduct synthesized by the post-oligomerization strategy to date. Adduct 8 is a major adduct in MC-treated tumor cells. However, in biomimetic reactions in vitro it is formed only in trace quantities. We present now definitive 1H NMR characterization of adduct 8. The present synthetic approach to the previously unavailable sister adduct 8 will provide a substrate to examine the biological and structural properties of 8 in parallel with its major groove adduct counterpart and the other minor groove guanine-N2 monoadducts of MC.