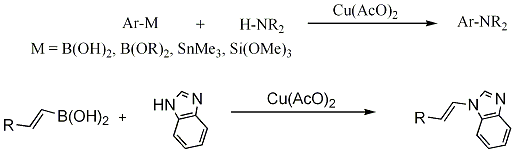Monday, May 19, 2008 - 3:40 PM
Science Building, Rm S-112 (Queensborough Community College)
272
COPPER-Promoted C-Heteroatom Bond Cross-Coupling Via Boronic Acids: Chan-Lam Coupling Reaction
Copper-promoted C-N/C-O bond cross-coupling between arylboronic acids and H-N/H-O containing substrates, a complementary reaction of Suzuki-Miyaura's palladium-catalyzed C-C bond cross-coupling, has recently emerged as an important and powerful synthetic methodology. The hallmark of this new coupling methodology is the mild conditions of the reaction, similar to the well-established condensation reactions to make the amide C-N bond � room temperature, weak base and in air. Since our initial reports, we have extended the methodology to include other organometalloids such as hypervalent arylsiloxanes and arylstannanes, in place of arylboronic acids, and general catalytic copper systems. The methodology is also applicable to N-vinylation with vinylboronic acids � the mildest method for performing N-vinylation. We would like to describe the development and mechanism of this important reaction and to contrast with Buchwald/Hartwig's palladium chemistry. Background: 1.Chan, Lam. Book chapter in �Boronic Acids� Hall, ed. 2005, Wiley-VCH, 205-240. 2. Lam, Clark, Saubern, Adams, Winters, Chan, Combs. Tetrahedron Lett. 1998, 39, 2941-2944. 3. Evans, Katz, West. Tetrahedron Lett. 1998, 39, 2936-2940. 4. Chan, Monaco, Wang, Winters. . Tetrahedron Lett. 1998, 39, 2933-2936.
Web Page: www.organic-chemistry.org/namedreactions/chan-lam-coupling.shtm