Wednesday, May 21, 2008 - 9:35 AM
Medical Arts Building, Rm M-142 (Queensborough Community College)
572
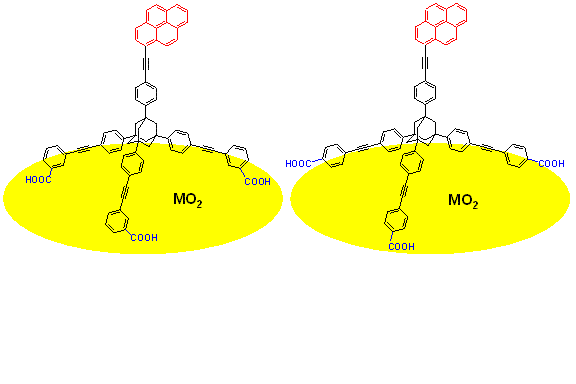
Aggregation Studies of Tripodal Linkers on Semiconductor Surfaces
Tripod shaped adamantane derivatives carrying a pyrene sensitizer and three carboxylic acid binding groups (in meta or para positions) with foot print size of 2.7nm2 were synthesized to study the effect of the footprint size on the binding and aggregation processes. The photophysical properties were studied in solution and bound to metal oxide surfaces. The UV-vis absorption and fluorescence emission spectra in solution of the pyrene unit were red-shifted due to the extended ∏-conjugation. Higher extinction coefficients and near unity quantum yields were also observed. Binding to base pre-treated anatase TiO2 films resulted in quenching of the fluorescence indicating electron injection into the semiconductor. Tripods with large footprint, by design, should isolate the pyrene molecule from the nearest neighbors and avoid the formation of excimers on ZrO2 films (ZrO2 is an insulator and the fluorescence is not quenched). However, we observed excimer emission at 505 nm, suggesting contacts between pyrene chromophores from adjacent nanoparticles and necking regions. The effect of aggregation of pyrene chromophores on ZrO2 films studied as a function of surface coverage, solvents, temperature, dyeing times and addition of additives will be discussed.