Tuesday, May 20, 2008 - 2:35 PM
Library Building, Rm LB-15 (Queensborough Community College)
477
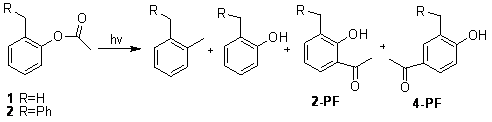
Photo-Fries Rearrangements of O-Cresyl Acetates in Isotropic Solutions and in Polyethylene Films. the Role of Reaction Cavities
The photo-Fries rearrangements of o-cresyl acetate (1) and 2-benzylphenyl acetate (2) have been investigated in two unstretched and stretched polyethylene (PE) films and in isotropic solutions. For example, irradiation of 1 yields principally o-cresol, o-xylene, 3-methyl-2-hydroxyacetophenone (2-PF) and 3-methyl-4-hydroxyacetophenone (4-PF) in isotropic solvents, but 2-PF dominates in PE films, especially in stretched PE films. The influence of variables, such as the degree of film crystallinity, film free volume, and the unstretched/stretched state of the films, on the rearrangements has been explored. The selectivity of the photo-Fries rearrangements of 1 is higher in PE films than in isotropic solutions, and increases with degree of crystallinity of the films and upon film stretching. o-Cresol, a product from cage-escape of the initially generated aryloxy and acyl radical pairs, and o-xylene, a product from concerted decarboxylation, are formed in yields that depend upon the nature of the films. These and other results will be discussed.
We thank the U.S. National Science Foundation and the China Scholarship Council for their support of the research.