Sunday, May 18, 2008
Student Union Building, Upper (Queensborough Community College)
156
Development of 5HT2A Antagonists Based on the Aporphine Alkaloid Nantenine
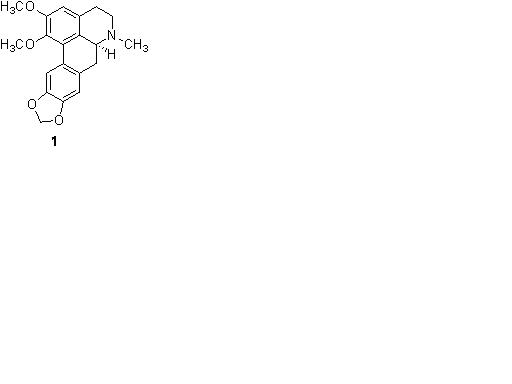
MDMA (3,4-methylenedioxy-N-methylamphetamine), most commonly known by the street names Ecstasy, E, X, or XTC is a semi-synthethic derivate of the phenethylamine family. MDMA acts directly on a number of receptors, including á1-adrenergic and 5-HT2A receptors. Use of “Ecstasy” is accompanied by several adverse physiological effects including the development of hyperthermia, organ failure and death in extreme cases. Cognitive impairment has also been reported in “Ecstasy” users and there is evidence that the drug has addictive properties. Nantenine (1) is an aporphine alkaloid isolated from the Japanese fruit of Nandina domestica. Nantenine shares structural similarities to MDMA and is an antagonist at serotonin 5-HT2A and adrenergic á1 receptors. Up to the present time, very little structure-activity relationship (SAR) studies have been performed on nantenine in relation to its antagonist activity at these receptors. It is the goal of this project to synthesize analogs of nantenine and to explore the functional group and spatial characteristics of the molecule which are required for antagonist activity at 5HT2a receptors. The analogs will also be evaluated for their ability to block the discriminative stimulus effects of MDMA in mice. These combined efforts will allow for the development of potent 5HT2a antagonists based on the nantenine core structure through iterative synthesis and in vitro screening and will also identify molecules with potent in vivo MDMA antagonist activity. Our efforts towards synthesis of nantenine analogs to date will be presented.