Monday, May 19, 2008
Student Union Building, Upper (Queensborough Community College)
328
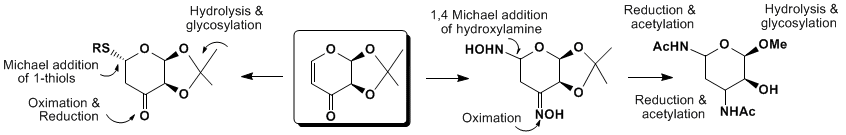
NEW APPROACH to 3, 5-DIAMINO-SUGARS from 4, 6-DIDEOXY-1, 2-O-ISOPROPYLIDENE-D-Glycero-PENT-4-ENOPYRANOS-3-ULOSE
Our stereoselective enones functionalization approach was first developed by the utilization of the Michael addition of reactive thiols (SugSH) to various carbohydrate enones (levoglucosenone (1) [1-4] and expanded to iso-levoglucosenone (2) [5]) and new functional L-arabinose enone [6]. These approaches constitute a new entity in the variety of new 1, 4-, 1-2-thio-linked S-disaccharides previously not easily available as well as rare 3-amino-sugars as components of various classes of carbohydrate antibiotics. In this context, extension of our synthetic strategy to amino functionalized analogs as biological targets requires stereoselective fuctionalizations at C-3, or C-5 positions respectively. Convenient functionalizations of the C-3 keto by oximation and C-5 by 1, 4 Michael addition of hydroxylamine to C-5 of enone moiety, followed by stereoselective reduction of oxime/hydroxylamine functionality with 9-BBN produce new 3, 5-diamino analogs. These new analogs of 3, 5 diaminosugars are convenient precursors suitable for further peptidomimetics construction that will be presented and discussed.