Wednesday, May 21, 2008 - 9:25 AM
Medical Arts Building, Rm M-136 (Queensborough Community College)
585
Oxazolone Cycloadducts as Versatile Scaffolds for Alkaloid Synthesis
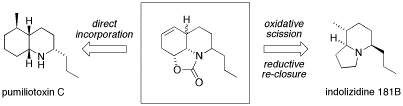
We have recently developed a series of intramolecular Diels-Alder reactions with oxazolone as the dienophilic species. These initial cycloadducts offer functionalized scaffolds reminiscent of several key alkaloid structural motifs, each well-suited to further elaboration as required. Application to the synthesis of several bioactive alkaloid targets is currently underway. For example, the common n-propyl cycloadduct shown features not only the decahydroquinoline framework of pumiliotoxin C, but could also allow entry to the corresponding indolizidine system via a novel reorganization strategy.