Sunday, May 18, 2008 - 9:55 AM
Science Building, Rm S-112 (Queensborough Community College)
25
A New Type of Karplus Equation? Dependence of Phosphorus-Hydrogen Coupling Constants on Lone-Pair Conformation
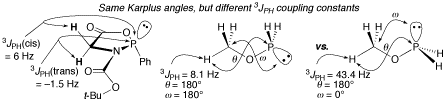
In contrast to literature reports of a Karplus-type curve that correlates 3JPH with phosphorus-hydrogen dihedral angle, the recently-reported glycine-derived phosphorus heterocycle shown has two hydrogen atoms on the ring with identical PNCH dihedral angles but measured coupling constants of ~6 Hz and 1.5 Hz. DFT calculations suggested that the smaller coupling constant is negative. Experimental evidence of the sign of the coupling constant was obtained by analysis of the ABX NMR spectrum of a new glycine-derived N-p-toluenesulfonyl analogue. Calculation of the phosphorus-hydrogen coupling constants of (CH3)nYPH2 (Y = C, n=3; N, n=2; O, n=1) both as a function of the PYCH dihedral angle (θ) and the lone pair-PYC dihedral angle (ω) showed similar θ,ω surfaces for 3JPH, and a range of 3JPH from �4.4 Hz to +51 Hz. While a modified Karplus equation as a function of θ and ω can be derived and provides qualitative agreement, no single equation can suffice to provide quantitative predictions for a range of atoms. DFT calculations provide a simpler means to accurately determine 3JPH.