Monday, May 19, 2008
Student Union Building, Upper (Queensborough Community College)
308
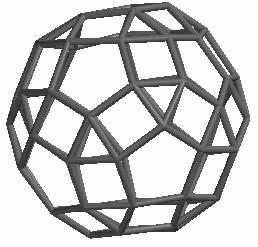
Theoretical Calculations of C60 Isomers: A New Stable? or Unstable? Isomer C60[3,4,5]
The well known and stable isomer of C60 consists of five and six-membered aromatic rings. We have successfully built computer models of two other C60 isomers; a previously reported C60[3,10] isomer and, to our knowledge, a previously unreported C60[3,4,5]. As shown below, this isomer consists of 3, 4 and 5 membered rings. Semi-empirical calculations indicate that this isomer is very unstable with a heat of formation of 5,500 kcal/mol compared with C60[5,6] which is 810 kcal/mol.