Sunday, May 18, 2008
Student Union Building, Upper (Queensborough Community College)
143
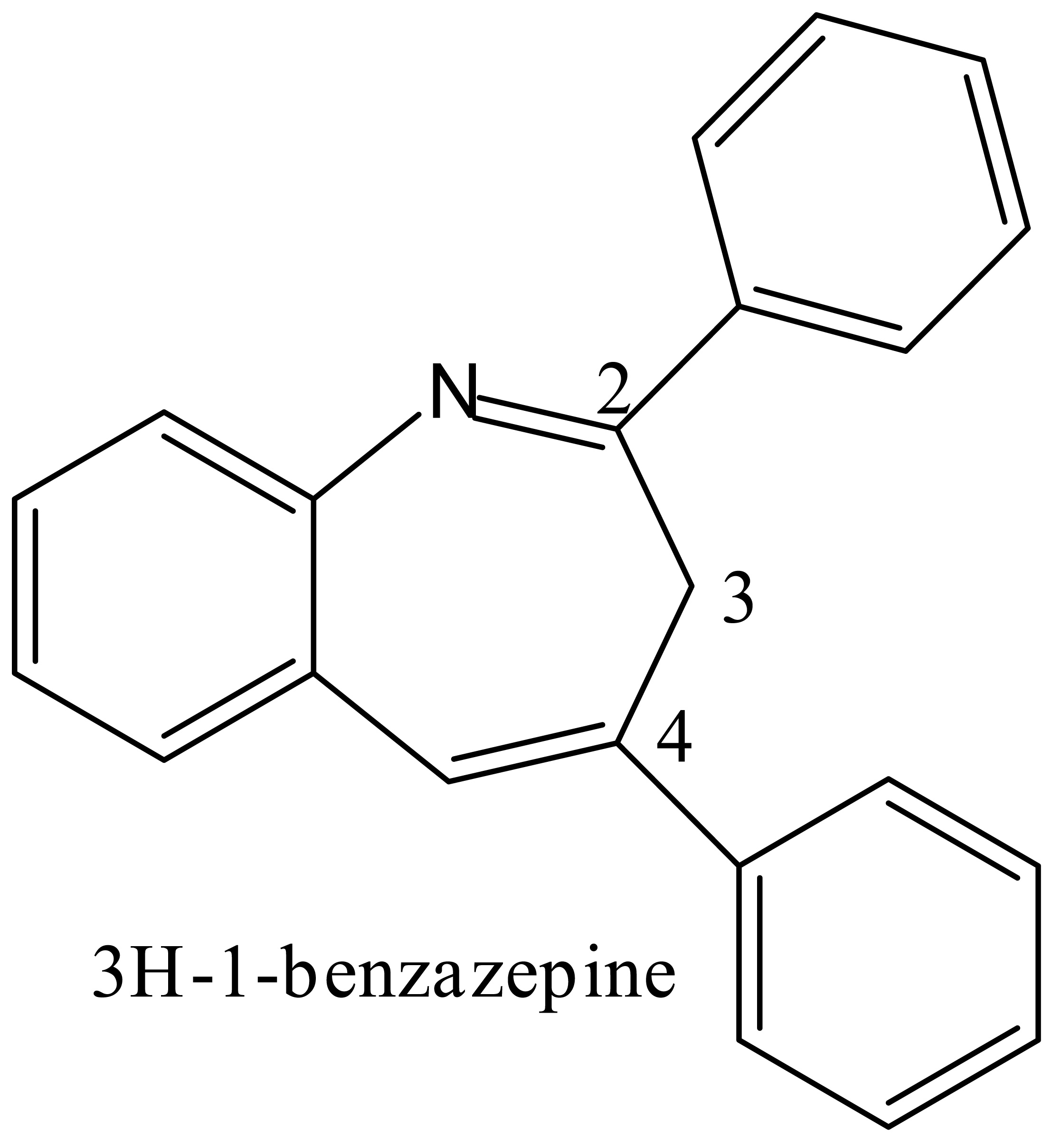
NMR Studies on the Conformational Exchange in 1-Benzazepines
Benzazepines are a pharmacologically important group of compounds used in the treatment of HIV and many other illnesses. A facile synthesis of 3H-1-benzazepine was reported by one of us recently. Energy minimization by MM2 forcefield shows that the molecule adopts a conformation where the C3 is out of the plane of the heterocyclic ring system along with one of the two phenyl rings attached to the C2 and C4-positions. At room temperature, the NMR spectrum of 1-benzazepine shows only one resonance at 3.4 ppm for the methylene protons at C3, suggesting that the molecule equilibrates rapidly between two conformations (C3 above or below the plane). At -65 oC, the exchange is slow and two separate resonances are observed at 4.5 and 2.2 ppm. The low temperature experiment is explored to assign the proton and carbon NMR spectra of 1-benzazepine derivatives. We will also describe calculations to measure the activation barrier for the conformational equilibrium.