Sunday, May 18, 2008
Student Union Building, Upper (Queensborough Community College)
158
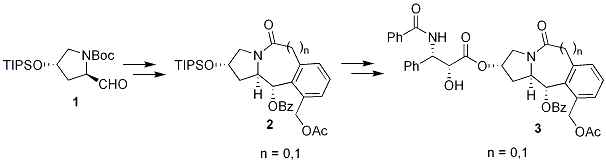
Design and Synthesis of De Novo Paclitaxel Mimics Based on REDOR-Taxol Structure
Paclitaxel (Taxol®) currently serves as one of the most important drugs in cancer chemotherapy. The complex baccatin core could be mimicked by a structurally simpler scaffold that retains its essential features based on the binding conformation of paclitaxel in microtubule. We designed novel baccatin-free paclitaxel mimic 3 that can take the “REDOR-Taxol” structure at the binding site in beta-tubulin. Fused 5-6-6 and 5-7-6 tricyclic ring systems 2 have been synthesized from hydroxyproline derivative 1. Molecular modeling studies, synthesis and biological evaluation of these novel paclitaxel mimics will be presented.