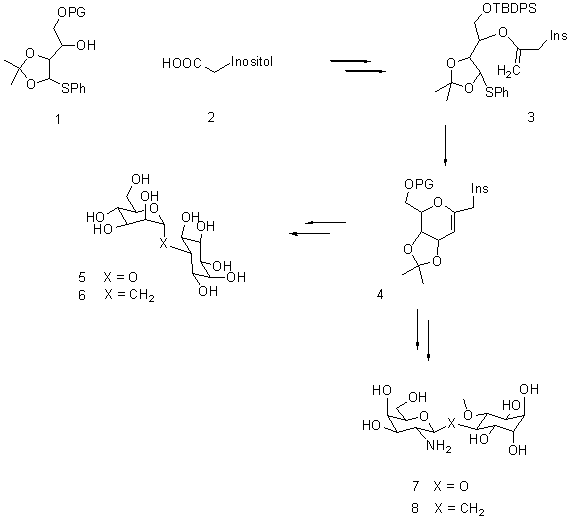Monday, May 19, 2008
Student Union Building, Upper (Queensborough Community College)
314
Synthesis of C-Glycosides of Glycoinositols Pseudodisaccharides
Glycosylinositols make up the framework of the glycosyl phosphatidyl inositol (GPI) anchor that covalently links certain proteins to the outer leaflet of eurkayotic cell membranes. GPI's are also attached to high mannose oligosaccharides in the cell wall of mycobacteria. Glycosylinositols are also believed to function as secondary messengers in important cellular processes, such as the mediation of insulin action. C-linked glycoinositols because of their greater hydrolytic stability and different conformational behavior compared to their native O-glycosides have attracted interest as biomechanistic probes.
The synthesis of 6 and 8, the C-glycosides of 6-O- (a-mannosyl)-myo-inositol 5 and 4-O- (2-amino-2-deoxy-b-D-galactopyranosyl)-3-O-methyl-D-chiro-inositol 7, will be presented. Pseudo-disaccharide 5 is an important subunit of microbacterium cell wall, and is putative insulin mediator. The key step in the synthesis of C-glycosides 6 and 8 is the formation of glycals like 4 from enol-ether via an oxocarbenium ion-alkene cyclization. Enol ether was prepared by Tebbe olefination of the ester derived from thio-acetal 1 and acid 2.