Tuesday, May 20, 2008
Student Union Building, Upper (Queensborough Community College)
519
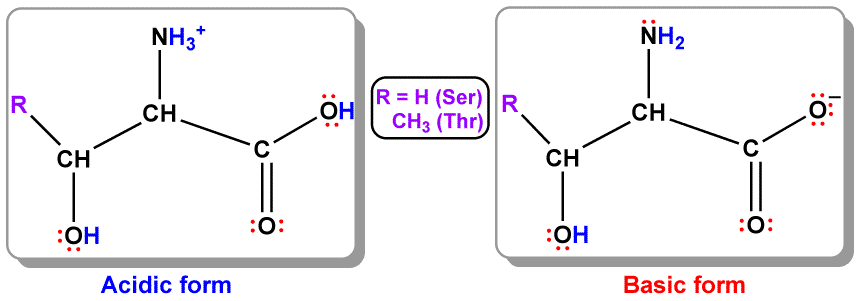
Theoretical Studies of Basic and Acidic Serine and Threonine – An Exploration of Hydrogen Bonding Patterns
One of the greatest challenges posed to chemists by amino acids is determining their preferred conformations, due to their intrinsic flexibility that gives rise to various intra- and intermolecular hydrogen bonding interactions. Most of these conformers are close in energy, within less than 5 kcal/mol. The specific hydrogen-bonding pattern controls the structure of these amino acids in an enzyme, and can significantly affect its activity. Mutation studies demonstrated that altering a single amino acid could reduce or even eliminate the activity of that particular enzyme. By employing theoretical tools, such as, the hybrid Hartree-Fock (HF) and density functional theory DFT), B3LYP/6-31+G(d,p) method implemented in the Gaussian 03 program, we determined the major conformers of serine (Ser) and threonine (Thr) in acidic and basic pHs. The acidity of the medium greatly influences hydrogen bonding patterns, and thus the stability of the resulting conformers. We will also discuss the theoretical chemical shifts of these molecules, and compare them to available experimental data.