Tuesday, May 20, 2008 - 9:10 AM
Medical Arts Building, Rm M-146 (Queensborough Community College)
392
A Computational Study of Changing Intramolecular Interactions in Serine and Threonine, at Different Phs
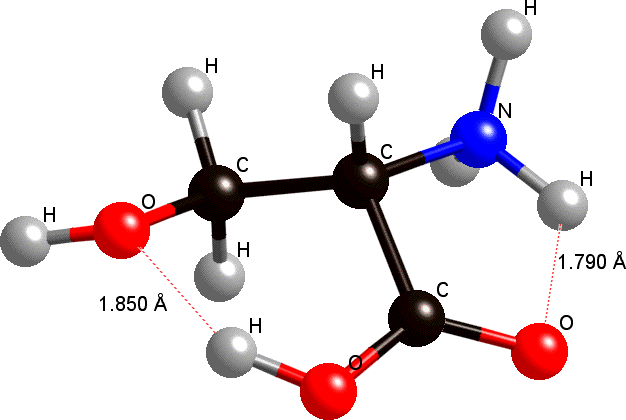
Amino acids are excellent targets for theoretical investigations, because of their small sizes and remarkable role in our lives. Yet, their flexibility leads to multiple conformations that stem from the many possibilities for intra- and intermolecular hydrogen bonding interactions, which control secondary and tertiary structures of proteins and affect enzymatic activity. We analyze conformers of serine (Ser) and threonine (Thr), in different pH, using DFT methods. We also compute chemical shifts, and compare them to currently available experimental data. Hydrogen bonds' strength, as well as the strain they impose on these systems are strongly influenced by pH. By examining these intrinsic bonding elements in Ser and Thr, we aim to identify similar hydrogen bonding motifs in complex biological systems.