Sunday, May 18, 2008
Student Union Building, Upper (Queensborough Community College)
159
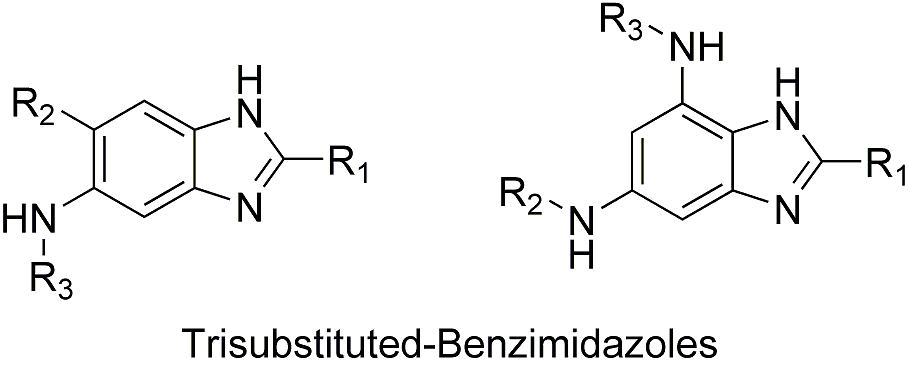
Synthesis and SAR Study on Novel Benzimidazoles for Antituberculosis Drug Discovery, Targeting FtsZ
FtsZ, a tubulin homologue, is a ubiquitous bacterial cytoskeletal protein. In the presence of GTP, FtsZ polymerizes to form a highly dynamic helical Z-ring at the mid cell eventually leading to septation. Inhibition of FtsZ results in the absence of septation contributing to arrested bacterial growth. Therefore, we hypothesized that FtsZ-inhibitors can be developed into antibacterial agents possessing novel mechanism of action. Accordingly, we designed and synthesized a library of novel trisubstituted-benzimidazoles through rational and systematic design. A number of these compounds exhibited < 6 ìg/mL MIC99 activity in the preliminary screening against Mtb H37RV strain. Polymerization assay confirmed that these compounds inhibited Mtb-FtsZ in dose dependent manner. Synthesis, biological evaluation and SAR of novel benzimidazoles will be presented.