Sunday, May 18, 2008
Student Union Building, Upper (Queensborough Community College)
161
Design, Synthesis and Biological Evaluation of Novel Tumor-Targeting Conjugates
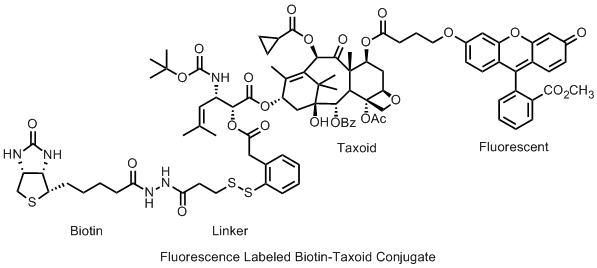
Novel biotin-taxoid conjugates have been evaluated for their efficacy in tumor-targeting drug delivery using confocal fluorescence microscopy. Recently, it has been shown that vitamin receptors, including biotin receptors, are overexpressed in various tumors. Thus, the biotin receptor is an excellent biomarker for tumor-targeting drug delivery via receptor-mediated endocytosis. A novel disulfide linker was designed and used for conjugating biotin and a taxoid, which would release the active taxoid inside tumor cells by the action of intracellular glutathione. Three conjugates were designed and prepared for process verification, i.e., biotin-conjugate internalization, disulfide bond cleavage, and active taxoid release, respectively. At each stage, a fluorescent or fluorogenic moiety was employed to visualize the progress. The design and synthesis of these biotin-taxoid-conjugates, as well as the cell-based evaluation of their tumor-targeting efficacy against L1210FR leukemia cell line, will be presented.