Tuesday, May 20, 2008
Student Union Building, Upper (Queensborough Community College)
535
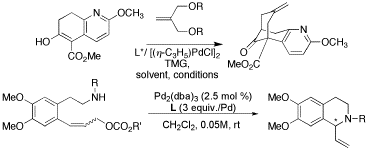
Novel Chiral Biphenol-Based Monodentate Phosphoramidite Ligands and Their Application to Asymmetric Allylic Substitution Reactions
A new class of monodentate phosphorus ligands based on the axially chiral biphenol have been designed and synthesized in our lab. These ligands possess fine-tuning capabilities through systematic modifications not only on the amine, alcohol or alkyl moieties, but also at the 3,3'-positions of the biphenol moiety. In this work, we have investigated applications of these ligands to allylic substitution reactions. The key step in the synthesis of Huperzine-A is a Pd-catalyzed bicycloannulation reaction. With excellent potential pharmaceutical applications of Huperzine A, we have chosen this double allylic alkylation reaction to test the efficacy our chiral ligand system. We have also chosen a Pd-catalyzed intramolecular asymmetric allylic amination reaction, which leads to the formation of chiral C1-substituted tetrahydroisoquinoline skeleton. We wil present our newly designed library of phosphoramidite ligands and their successful applications to these reactions