Sunday, May 18, 2008
Student Union Building, Upper (Queensborough Community College)
148
Novel Organosilane Chemistry for Approaches to Bioactive Ether Targets
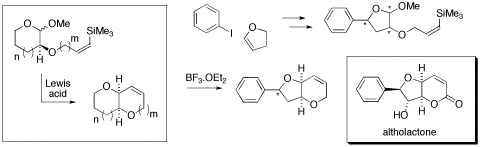
We have recently developed a general methodology for the construction of cis-fused bicyclic ether arrays. Our overall approach involves the intramolecular attack of a proximally tethered vinylsilane at an oxocarbenium moiety, generated in situ from the corresponding cyclic acetal under appropriate Lewis acid catalysis. This has allowed direct access to a variety of richly-functionalized cis-fused architectures, many of which are pertinent to specific bioactive natural products. One such example is the styryl lactones � a class of plant metabolites with wide-ranging cytotoxic properties. A concise synthesis of deoxyaltholactone analogs will be presented.