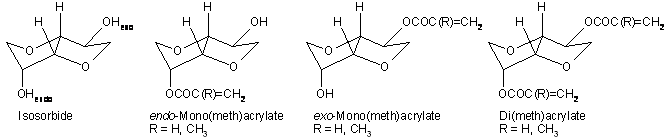Sunday, May 18, 2008 - 10:50 AM
Science Building, Rm S-112 (Queensborough Community College)
27
Regioselective Acylations of Isosorbide: Synthesis, Characterization and Radical Polymerization of Isosorbide (Meth)Acrylate Esters
1,4:3,5-Dianhydro-D-glucitol (isosorbide) is a chiral, thermally stable, biomass-derived bicyclic diol with a conformationally rigid structure made of two tetrahydrofuran rings fused in a cis junction. Catalytic hydrogenation of glucose to sorbitol followed by acid-catalyzed dehydration easily yields enantiopure isosorbide. Numerous isosorbide-containing thermoplastic polymers were prepared and characterized, showing high temperature performance, good impact strength, optical clarity, and biodegradability. The two hydroxy groups in isosorbide, one oriented exo- (C2) and the other endo- (C5), are diastereotopic and exhibit different reactivities. Herein, we report our efforts to efficiently and selectively synthesize isosorbide mono-exo, mono-endo and di- (meth)acrylates. The synthesis, characterization and polymerization kinetics of isosorbide (meth)acrylic esters are presented. The observed selectivities of various acylation procedures are discussed in relation to isosorbide stereochemistry and esterification mechanisms. All isosorbide (meth)acrylate esters were found to polymerize easily, and furnished hard, colorless, and infusible resins. Thermal properties of several isosorbide-containing acrylic polymers and co-polymers, i.e. glass transition temperature and decomposition behavior, are described. The carbohydrate-derived core could potentially make these monomers suitable for applications from chiral auxiliaries in cholesteric color filters, asymmetric reactions, or chromatography columns, to biodegradable building blocks in template-imprinting polymers.