Monday, May 19, 2008 - 9:20 AM
Science Building, Rm S-112 (Queensborough Community College)
180
Novel Penems Bearing a Bicyclic or Tricyclic Heterocycle on Methylidene Linkage as Broad Spectrum B-Lactamase Inhibitors and Their Mechanism of Inactivation
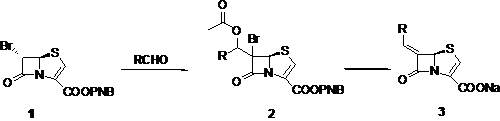
Inhibitors of bacterial b-lactamases in combination with other antibiotics have been used extensively in the clinical setting to over come resistance due to b-lactamase production.� Clinically used antibiotic/inhibitor combinations cover class-A producing pathogens, but have very little effect on class-C and ESBL producing organisms. However, methylidene penem inhibitors have shown promising broad-spectrum activity. In the present work, based on modeling studies, novel methylidene penems 3, having bicyclic and tricyclic heteroaryl moieties were designed and synthesized by a novel route. The structure activity relationship and mechanism of action of this class of compounds will be discussed.